In December 2018, the first AI-based medical device–the AI software to support discrimination between tumor/nontumor colorectal lesions using endoscopic images–was approved in Japan. Although nearly three years have passed since then, the number of AI-based medical devices approved in Japan is still low compared to Europe and the US.
However, if you were to ask me if there will be more artificial intelligence-based medical devices in Japan in the future, I would certainly answer Yes. This is because Japan has become one of the countries with the most advanced policies on artificial intelligence-based medical devices.
It is important to consider these three aspects when examining policies related to medical devices: research and development, regulatory aspects of efficacy and safety, and reimbursement pricing. Japan began studying these three points in January 2017 and, after continuous discussions, the cabinet approved the Regulatory Reform Implementation Plan in June 2021. This plan summarized various policies to address most of the issues to be tackled in the future.
Relevant initiatives in this journey from study to implementation plan are overviewed below.
June 2017: Ministry of Health, Labor and Welfare (MHLW) Round-Table Conference on Promoting the Use of AI in Healthcare
The report from the Round-Table Conference on Promoting the Use of AI in Healthcare identified six priority areas to be developed for AI utilization in healthcare and formulated a roadmap to 2020 for their realization: 1) diagnostic imaging support, 2) diagnostic and therapeutic support (including testing, disease management, and disease prevention), 3) surgical support, 4) nursing care and dementia, 5) genomic medicine, and 6) drug development.
December 2017: PMDA Scientific Committee Recommendations on Review of AI-Based Medical Devices
The PMDA’s Scientific Committee published “Issues and recommendations on AI-based medical diagnosis systems and medical devices,” which aimed to examine the characteristics and risks of AI-based medical devices and points to keep in mind when using them, and to help with regulatory review and consultation. (A summary version is also available from Advanced Biomedical Engineering.) These recommendations were prepared by the AI subcommittee of the Scientific Committee and contained the first mention of the concept of AI-based medical device review in Japan.
March 2019: Draft Review Guidance for AI Medical Devices (MHLW, NIHS)
The National Institute of Health Sciences (NIHS) published its “Report of the Review Working Group for Artificial Intelligence,” which summarized regulatory issues related to AI-based medical devices as part of the Next Generation Medical Devices and Regenerative Medicine Products Evaluation Index Project commissioned by the MHLW to the NIHS.
June 2019: Consortium for Accelerating AI Development in Healthcare Identifies Roadblocks by Development Stage (MHLW)
This report summarized, by clinical development stage, roadblocks to development of AI-based medical devices and announced the future direction of the project. This report identified these nine issues as roadblocks: 1) institutional review board (IRB), 2) informed consent, 3) annotation and labeling, 4) data transfer, standardization, and anonymization, 5) cloud computing and data storage, 6) clinical validation, 7) PMDA review and approval, 8) commercial deployment and updates, and 9) other areas. This consortium also assumed control of and responsibility for the Round-Table Conference on Promoting the Use of AI in Healthcare.
November 2019: Amendment of Pharmaceuticals and Medical Devices Act (PMD Act) to Establish PACMP
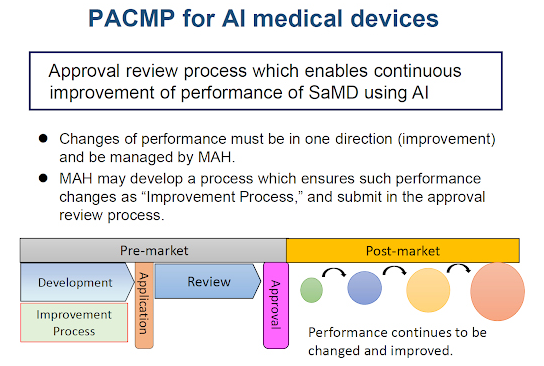
By Amendment of the Pharmaceuticals and Medical Devices Act, the Post-Approval Change Management Protocol for Medical Devices (PACMP) was established as an approval review system for medical devices including AI-based medical devices. In Japan, the PACMP is also commonly known as IDATEN (Improvement Design within Approval for Timely Evaluation and Notice).
Source: Tetsuya Kusakabe, PMDA: Regulatory Updates on Medical Devices in Japan: Amendment of Pharmaceuticals and Medical Devices Act (PMD Act), presented at IMDRF Web Conference, March 2021. Used with permission.
SaMD is an acronym for Software as a Medical Device.
June 2020: Consortium for Accelerating AI Development in Healthcare Establishes Process Chart for Resolving Roadblocks (MHLW)
To help facilitate the introduction of AI-based medical devices into clinical practice in Japan, the MHLW published a roadmap for the elimination of roadblocks in each of the nine stages of development until FY2022. MHLW is currently implementing various measures to resolve issues according to this roadmap.
November 2020: DASH for SaMD to be Developed (MHLW)
MHLW formulated and published its Drastic Reform of the Review of Advanced Medical Devices, including Programs, commonly known as “DASH for SaMD.” “DASH for SaMD” is an acronym for “DX (Digital Transformation) Action Strategies in Healthcare for SaMD (Software as a Medical Device)” and is designed to promote early commercialization of new SaMDs by identifying the seeds of SaMDs at an early stage, presenting the concept of review, centralizing the consultation service from development to reimbursement price, and establishing a review system and structure based on the unique characteristics of SaMDs. MHLW and PMDA have each established a new department specializing in SaMD to strengthen their systems toward achieving this goal.
March 2021: Start of Discussions to Clarify Concept of SaMD in Reimbursement Pricing
Chuikyo (the Japanese government’s reimbursement advisory council) and MHLW agreed to clarify the concept of reimbursement pricing for SaMD in the discussion for the 2022 revision of government reimbursement policy. This discussion is still in progress.
June 2021: Regulatory Reform Implementation Plan for Japan
The cabinet approved the Regulatory Reform Implementation Plan and announced policies to further accelerate the development and commercialization of SaMD, including AI-based medical devices, in collaborative industry-regulatory development.
Conclusions
SaMDs have only been subject to pharmaceutical regulations in Japan since the 2014 enforcement of the law revised in 2013. Since then, discussions on systems and policies have rapidly progressed in tandem with the development of AI against the backdrop of major challenges coming soon to Japan’s healthcare system. For example, it is known that Japan’s population will decline rapidly, potentially creating a shortage of workers that is expected to have a major impact on healthcare in Japan. Artificial intelligence can help move the burden of many practical tasks from skilled physicians to clinical engineers, nurses, and other staff.
In a related effort, the Roundtable Conference on the Promotion of Venture Businesses for Medical Innovation report compiled by a private panel of the MHLW in July 2016 established three principles for specific measures to promote medical business ventures: “from regulation to cultivation,” “from caution to speed,” and “from macro to micro.”
Since then, the overall policy goal for innovative medical product review and approval has changed from “catching up” (to the US and Europe) to providing an “incubation function” to help industry provide patients with cutting-edge medical technologies as quickly as possible, before other nations instead of after. Japan has developed a practical approach to AI-based medical devices, decided on priority areas, created a roadmap for their realization, and is implementing a five-year program in which industry, academia, and government work together to identify and resolve roadblocks to these efforts.
These are some of the reasons why my answer to the opening question is Yes.
CODEX北京科译翻译有限公司是一家专业提供生命科学翻译和本地化服务的公司,作为中国专业的生命科学翻译公司之一以及国内为数不多的通过国际ISO17100: 2015认证的医学翻译提供商,CODEX科译拥有更多的医学资深语言专家,能实现40多种语言对的互译,接受更高难度的医学领域的项目挑战,并在最短时间内适应以给出最合理的解决方案。保证最高质量,100%按时交付,高效、完善的售后保障是CODEX科译给客户的用心承诺。
CODEX is a leading provider of cross-border communications based in Beijing. With our exclusive database in life science, and ISO 17100:2015 certified, CODEX has the required expertise and experience in localization, documentation, and medical literature to deliver a full range of end-to-end content and consulting services in many languages. We closely work with clients to deliver solutions to the challenges of engaging markets, consumers, and regulatory and patent environments worldwide.